How adaptive plasticity evolves when selected against
Yes, that is the surprise title of Alfredo’s new paper in PLoS Computation Biology! When we discover examples of adaptive plasticity we usually think that it evolved because selection favoured it. But can adaptive plasticity evolve when selection favours non-plastic individuals?
Think of a population of insects that hatches, reproduces and dies twice every year: once in spring and once in autumn. Spring and autumn conditions may be very different, but plasticity could allow the insects to cope with both seasons. Yet, insects born in spring never experience autumn, and vice versa, so natural selection cannot directly favour plasticity. In fact, plastic individuals may do worse than individuals that are not plastic, which means plasticity could consistently be selected against.
This problem is similar to tuning the learning rate in Machine Learning. To teach a machine how to solve a problem, we give it a set of example questions we already know the answer to. Each time the machine gives us the wrong answer, we change its parameters to produce a new answer that is closer to the right one. If the examples share a consistent logic, the machine will eventually find this logic. But if we change the model’s parameters too much with every example shown, the machine will just repeat the solution for the last example, which prevents it from finding the underlying logic that connects the examples.
Just like in machine learning, organisms receive a set of questions in the form of environmental cues and need to provide the right answer in terms of a fit phenotype. Adaptive plasticity is the solution that produces the right phenotype for every environment. Organisms that see only one environment per lifetime could easily “forget” that plasticity is adaptive and instead only produce the right phenotype for their last environment. The offspring of insects that live in spring would be better matched to spring, even though they will have to deal with autumn conditions.
So when does learning theory predict that a machine will learn the logic of plasticity rather than the last solution? And do those predictions hold for a simple representation of evolution by natural selection? To find out, have a look at the paper!
Solved: the genetics of colour polymorphism in wall lizards
 A new paper in PNAS reveals that the orange and yellow ventral colour morphs in wall lizards is caused by regulatory changes in pterin and carotenoid genes. Interestingly, it seems like the alleles are old and occasionally shared between species. Miguel Carneiro and his team at CIBIO and Leif Andersson at Uppsala led the work, and we helped with the genome, samples, and other bits and pieces. The chromosome-scale genome assembly is of high quality and should become a useful resource – so just get in touch if you want to know if the wallies are right for you too!
A new paper in PNAS reveals that the orange and yellow ventral colour morphs in wall lizards is caused by regulatory changes in pterin and carotenoid genes. Interestingly, it seems like the alleles are old and occasionally shared between species. Miguel Carneiro and his team at CIBIO and Leif Andersson at Uppsala led the work, and we helped with the genome, samples, and other bits and pieces. The chromosome-scale genome assembly is of high quality and should become a useful resource – so just get in touch if you want to know if the wallies are right for you too!
A new take on the epigenetic signatures of prenatal stress
The conditions encountered in the womb can have life-long impact on health. It is usually assumed that this is because embryos respond to adverse conditions by programming their gene expression. In collaboration with Bas Heijmans and others, we propose that these effects also can be caused by selection on stochastic epigenetic variation. The paper is published in Cell Reports and there is also a press release.
The concept of fetal programming is based on the idea that embryos modify their physiology in response to the uterine environment. This may be good if conditions stay as predicted. But it may have negative health effects later in life if the conditions change. The concept of programming has been backed up by associations between adverse prenatal conditions and patterns of DNA methylation – an epigenetic mark that regulates gene expression.
Our simple insight is that the uterine environment does not need to induce changes in DNA methylation for this association between adverse prenatal conditions and DNA methylation to arise. In the early embryo, stochastic differences in gene expression can become stabilized by DNA methylation, resulting in embryos with different epigenomic profiles. Not programmed by the environment, these random differences in gene expression (and hence DNA methylation) may nevertheless provide some embryos with a survival advantage when conditions are harsh. In other words, adverse maternal conditions may impose selection on random variation in DNA methylation.
 Human embryo at 8-cell stage. (C) Yorgos Nikas/Science Photo Library
Human embryo at 8-cell stage. (C) Yorgos Nikas/Science Photo Library
We used a mechanistic simulation model to illustrate how selection reduce DNA methylation variance at loci that influence implantation success. The prediction fits very well with empirical data from offspring conceived during the Dutch Hunger Winter, a famine at the end of World War II. This makes selection on stochastic epigenetic variation a reasonable explanation for the epigenetic signatures of prenatal exposure to adverse conditions.
This new hypothesis is not only of academic interest. Fetal programming implies that the behaviour or physiology of the mother causes the offspring phenotype to change. In contrast, a selective process does not bring new phenotypes into being, it changes the distribution of already existing variants. This difference may influence which preventive strategies and treatments that are most likely to be effective. That different mechanisms can be responsible for the same pattern is also relevant to society. As Sarah Richardson has pointed out, careless discussion of epigenetic research on how early life affects health across generations could be harmful.
Developmental Bias and Evolution at Santa Fe
The third and final workshop funded by our EES grant took on the role of developmental bias in evolution. Like the meeting in February, Santa Fe greeted us with snow, jetlag, and huevos rancheros for breakfast.
The workshop showcased the extraordinary breadth of approaches used to understand if, and how, developmental processes direct evolution. Ecologists, developmental biologists, and palaeontologists explained what data are out there and what they mean. Theoretical biologists and computer scientists convincingly showed that the role of development not only can be formulated in precise terms, but that there are predictions ready to be tested. Judging from the work presented, we can look forward to some very interesting studies doing just that in the near future.
As there should be, there was both unity and spirited disagreement – with views ranging from development as constraint to development as the main driver of evolution. History and philosophy of biology again proved useful to understand this diversity. While debate over the role of development in evolution is as old as evolutionary science, contemporary research really does seem to bring new insights – conceptually, theoretically and empirically.
One way to think of developmental bias is through the use of morphospaces. The morphospace of several groups of reptiles (including dinosaurs) that lived around 230-200 million year ago, using two (undefined) shape characters. Reproduced from the Palaeobiology Research Group of the University of Bristol, UK
Origin-Of-Life researcher Wim Hordijk kindly summarized his views of the meeting on this blog. The Lund crew – Tobias, Alfredo, Nathalie, Reinder, and Illiam – are very grateful to the SFI for hosting, and the organisers for giving us a lot of things to think of. Next up – the Evolution Evolving meeting at Cambridge. See you there!
Another bad day for anticipatory maternal effects
A new study on water fleas, headed by Reinder and Alex, suggests that a classic example of adaptive maternal effects is not as adaptive as we might have thought.
Some years ago, Tobias, Sinead and Shinichi did a meta-analysis that seemed to undermine the idea that maternal effects are designed to transfer information about the local environment. Reviewers and editors did not really want to hear that, and the paper proved somewhat difficult to publish (but has been well received and cited). But one ‘anticipatory maternal effects’ appeared well supported: water fleas exposed to toxic cyanobacteria produced offspring that were more tolerant to the toxin.
So, to understand how this maternal effect evolves, Reinder and Alex challenged water flea mothers and offspring with cyanobacteria that produce the toxin microcystin. But rather than just expose the animals throughout their lives, they also exposed mothers at different times during her life. The rationale is that maternal effects that have been selected to transmit information are expected to behave like signals – triggered and delivered by a system well designed to pass on information with limited cost to the receiver.
It turned out that offspring indeed were somewhat better able to handle the toxin when their mothers had been exposed late in their lives. In other words, tolerance or resistance can be transmitted to the next generation through non-genetic means. But there was not much to suggest that the mechanism has been selected to transmit information about the presence of cyanobacteria. Instead, our experimental evidence – along with a meta-analysis of Daphnia research – fits better with a model of passive transfer of tolerance rather than an anticipatory maternal effect. Again, reviewers and editors were not very happy to hear this, and this paper also proved somewhat difficult to publish.
We should not be too surprised of these results, however. Not all plastic responses that allow organisms to cope with stress are properly seen as adaptations to cope with the stressor.
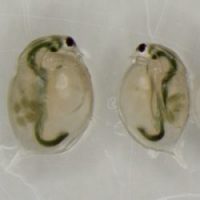
Genetically identical sisters, raised in two different environments. Daphnia raised in the microcystin treatment (on the right) displays smaller body size and carries less eggs than her sister from the control treatment
We can think of these maternal effects as – in Mary-Jane West-Eberhard terms – examples of phenotypic accommodation that have not yet been followed by genetic accommodation.
But while our results question if there really has been selection for maternal transfer of information about microcystin exposure, the study is still not quite conclusive. Two kinds of follow-up studies would be particularly informative.

Endless replications
The first is to contrast responses of populations of water fleas with different evolutionary histories of exposure. This kind of comparison can effectively reveal if plastic responses in naïve animals become fine-tuned over evolutionary time (see a nice example from house finches here).
The second is to reveal the mechanism of the maternal effect. This would not only tell us ‘how it works’, but – keeping the comparison with signals in mind – whether or not the mechanism has properties that we expect of systems designed to transmit information.
We have our ideas about what is going on, and Alex has just completed a set of experiments that can tell us more. In the meantime, we hope that this paper will inspire more studies to look at maternal effects as a window into the evolutionary transition from stress-induced responses to local adaptation. To get started, please see here and here.